Theme: The Developmental and Regulatory Landscape of Orphan Drugs in Perspectives of Gaming and Abuse
Orphan Drugs 2017
As of now the orphan medication spending in the United States is about $15.9 billion in 2013 and over $30 billion in 2016, an expansion from 4.8 percent of aggregate pharmaceutical spending to 8.9 percent. With the exponential developing business sector of orphan medications and effective past meetings in Europe and USA we are sorting out the Annual Congress on Emerging Orphan Drugs and Rare Diseases , October 30-November 01, 2017 at San Antonio, USA
Orphan Drugs 2017 will thoroughly talk about most recent advancements and market inclines in vagrant medications and uncommon maladies over the accompanying tracks
Track 1: Orphan Products
Orphan medications are therapeutic items planned for determination, counteractive action or treatment of life-undermining or intense illnesses or disarranges that are uncommon. An ailment or turmoil is characterized as uncommon in Europe when it influences under 1 in 2,000 natives. These medications are called "orphan" in light of the fact that under typical economic situations the pharmaceutical business has little enthusiasm for creating and showcasing items expected for just a little number of patients. For medication organizations, the amazingly high cost of putting up a therapeutic item for sale to the public would not be recuperated by the normal offers of the item. Therefore the potential market for new medication treatment is additionally little and the medication organizations industry would really bring about a money related misfortune. In this manner governments and uncommon illness persistent associations, for example, EURORDIS advocate for monetary motivating forces to urge medicate organizations to create and showcase prescriptions for uncommon sickness treatment.
Related Conferences:
International Conference and Exhibition on Nano medicine and Drug Delivery, May 29-31, 2017 Osaka, Japan. 2nd International Conference and Exhibition on Marine Drugs & Natural Products, June 15-17, 2017 | London, UK.10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK.5th International Conference and Exhibition on Pharmacology and Ethnopharmacology, March 23-25, 2017 Orland, USA.6th Global Experts Meeting on Cardiovascular Pharmacology and Cardiac Medications, April 13-14, 2017 Dubai, UAE.
Related Societies:
Track 2: Development Trends and Strategies on Orphan Drugs
With blockbuster drugs going off licenses the income misfortune to be acquired by numerous pharma organizations is relied upon to be enormous. Orphan Drugs are claimed to be a beneficial substitute for them to minimize the effect of income misfortune. Motivators granted by FDA and EU commission are an additional inspiration and help towards creating vagrant medications. Generally there has been seen an expanded improvement of vagrant medications by the pharma organizations and picking up advertising endorsements with market eliteness. The new plan of action of vagrant medications could offer an incorporated human service arrangement that empowers pharma organizations to create more up to date territories of therapeutics, determination, treatment, observing, and tolerant support.
Related Conferences:
3rd Annual Congress on Rare Diseases and Orphan Drugs, October 30-November 1, 2017 San Antonio, USA; World Orphan Drug Congress USA, April 19-21, 2017, Washington DC, USA; Drug Discovery 2017, March 27–28 2017, London, United Kingdom; International Conference on Rare Diseases & Orphan Drugs, October 19-22, 2017, Cape Town, South Africa; European Biopharma Congress, November 16-17, 2017 Vienna, Austria.
Related Societies:
Track 3: Challenges in Rare Diseases
With strict rules executed for unprecedented infirmities it is to a great degree difficult to participate in phenomenal sickness ask about on a subject without the assistance of a clinician moreover constraining the clinical trials limited keeping in mind the end goal to have a trust of filling them. Generally there is a deficiency of benefits for research on exceptional conditions of the disseminates included with a little market degree and nonattendance of existing written work and experienced specialists are some huge challenges. 452 drugs and inoculations being produced for remarkable diseases use stimulating new coherent and particular data. A strong bit of the medications, which offer look for after those anguish from one of the right around 7,000 unprecedented diseases, address imaginative better ways to deal with target affliction.
Related Conferences:
Orphan Drugs and Rare Disease Europe, 15 -16 May, 2017 Berlin, Germany. Drug Discovery 2017, 27–28 March 2017 London, United Kingdom. Orphan Drugs Summit, 21-22 September, 2017 Netherlands. 7th International Meeting on Pulmonary Rare Disease and Orphan Drugs, 24-25 February, Milan, Italy. 11th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, Feb 27- 28, 2017 Amsterdam, Netherlands.10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK.
Related Societies:
Track 4: Market Analysis for Orphan Drugs
With 30 million Americans experiencing 7000 rare illnesses the market for Orphan medications is developing exponentially. In addition according to the characterized laws vagrant medications appreciate a half duty acknowledge on R&D costs for $30m gifts for each financial year to do Phase I to Phase III clinical trials. The ascent of vagrant medication numbers in USA is by 12% enrolling 291 quantities of vagrant medications. Be that as it may, the development is significantly more quickened in Europe with a staggering ascent by 62% enlisting 201orphan drugs. Comprehensively vagrant medications deals estimate expects a $178bn piece of the pie with more than 11.7% piece of the overall industry and 20.2% overall medicine share by 2020.
Related Conferences:
Drug Discovery 2017, 27–28 March 2017 London, United Kingdom. Orphan Drugs Summit, 21-22 September, 2017 Netherlands.7th International Meeting on Pulmonary Rare Disease and Orphan Drugs, 24-25 February, Milan, Italy.11th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, Feb 27- 28, 2017 Amsterdam, Netherlands.10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK.5th International Conference and Exhibition on Pharmacology and Ethnopharmacology, March 23-25, 2017 Orland, USA.6th Global Experts Meeting on Cardiovascular Pharmacology and Cardiac Medications, April 13-14, 2017 Dubai, UAE.
Related Societies:
Track 5: Orphan Drugs Pricing and Reimbursement
Evaluating and repayment of vagrant medications is of most extreme significance to strategy creators, social insurance experts, lawmakers, industry pioneers, payers, pharma analytical market and patients. The evaluating are driven by variables like market selectiveness, less option wellbeing innovations, outsider payers, tremendous R&D costs should be recovered from generally less number of patients. There untruths earnestness for straightforward and proof based approach towards orphan drugs valuing and repayments. The approach is required to include relative adequacy, cost-viability, cost structure, monetary suitability of rare medications with an aim towards illuminating valuing and repayment choices.
Related Conferences:
7th European Bio-similars Congress May 15-17, 2017 Munich, Germany. 6th World Pharmacists & Clinical Pharmacy Annual Congress, May 22-23, 2017 | Chicago, USA. International Conference and Exhibition on Nano medicine and Drug Delivery, May 29-31, 2017 | Osaka, Japan. 2nd International Conference and Exhibition on Marine Drugs & Natural Products, June 15-17, 2017 | London, UK.
Related Societies:
Track 6: Types of Rare Disease
Rare diseases are such diseases or disorders which have been reported in less than 5 people per 10,000 people. Rare disease can be classified based on Arthrogryposis, Rare cancers, Cystic fibrosis, rare infectious diseases, Intersex and medicine, Mesothelioma, People with caudal regression syndrome, People with tetra-amelia syndrome, Progeroid syndromes, Supernumerary body parts, Tay–Sachs disease. Rare Diseases are often manifested by anatomical or physiological deformities in the patients.
Related Conferences:
11th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, Feb 27- 28, 2017 Amsterdam, Netherlands. 10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK. 5th International Conference and Exhibition on Pharmacology and Ethnopharmacology, March 23-25, 2017 Orland, USA. 6th Global Experts Meeting on Cardiovascular Pharmacology and Cardiac Medications, April 13-14, 2017 Dubai, UAE. International Conference and Exhibition on Pharmaceutical Development and Technology, April 24-26, 2017 Dubai, UAE. 10th Asia-Pacific Pharma Congress, May 08-10, 2017 Singapore City, Singapore.
Related Societies:
Track 7: Overview on Rare Diseases
An orphan medication is a pharmaceutical specialist that has been produced particularly to treat an uncommon therapeutic condition, the condition itself being alluded to as a vagrant sickness or uncommon infection. Vagrant medications for the most part take after an indistinguishable administrative advancement way from whatever other pharmaceutical item, in which testing concentrates on pharmacokinetics and pharmacodynamics, dosing, security, wellbeing and adequacy. In any case, some factual weights are decreased with an end goal to keep up improvement force. The improvement of vagrant medications has been fiscally boosted through US law by means of the Orphan Drug Act of 1983.
Related Conferences:
World Orphan Drug Congress USA, 19 - 21 April 2017; Washington, D.C.2nd World Congress on Rare Diseases and Orphan Drugs, June 29-30, 2017 London, UK. Orphan Drugs and Rare Disease Europe, 15 -16 May, 2017 Berlin, Germany. Drug Discovery 2017, 27–28 March 2017 London, United Kingdom. Orphan Drugs Summit, 21-22 September,2017 Netherlands.7th International Meeting on Pulmonary Rare Disease and Orphan Drugs, 24-25 February, Milan, Italy.
Related Societies:
Track 8: Genetic Rare Diseases
A hereditary issue brought about by at least one anomalies in the genome, particularly a condition that is available from birth (inherent). Most Genetic issues are very uncommon and influence one individual in each few thousands or millions.
Hereditary clutters might be genetic, passed down from the guardians' qualities. In other hereditary issue, deformities might be brought about by new transformations or changes to the DNA. In such cases, the imperfection may be passed down on the off chance that it happens in the germ line. A similar malady, for example, a few types of malignancy, might be brought on by an acquired hereditary condition in a few people, by new transformations in other individuals, and chiefly by natural causes in other individuals.
Related Conferences:
7th European Bio-similars Congress May 15-17, 2017 Munich, Germany. 6th World Pharmacists & Clinical Pharmacy Annual Congress, May 22-23, 2017 | Chicago, USA. International Conference and Exhibition on Nano medicine and Drug Delivery, May 29-31, 2017 | Osaka, Japan. 2nd International Conference and Exhibition on Marine Drugs & Natural Products, June 15-17, 2017 | London, UK.
Related Societies:
Track 9: Clinical Research on Rare Diseases
Uncommon ailments are oftentimes life-undermining or incessantly incapacitating and the effect on the personal satisfaction of influenced patients. Be that as it may, sedate advancement for these conditions has been restricted by an absence of comprehension of the basic instruments of infection and the relative inaccessibility of subjects for clinical trials.
Rare infections may include incessant disease, incapacity, and frequently sudden passing. They are intricate, and frequently with insufficient or no treatment, in this way speaking to a lopsided share of social insurance spending. Patients with uncommon illnesses are regularly misdiagnosed or are undiscovered. Few medication organizations direct research into uncommon maladies since it is hard to recuperate the expenses of creating medicines for little, geologically scattered populaces. To propel medicinal research on uncommon ailments, an examination arrange encourages cooperation, enrolment in studies and trials, and sharing of information.
Related Conferences:
World Orphan Drug Congress USA, 19 - 21 April 2017; Washington, D.C.2nd World Congress on Rare Diseases and Orphan Drugs, June 29-30, 2017 London, UK. Orphan Drugs and Rare Disease Europe, 15 -16 May, 2017 Berlin, Germany. Drug Discovery 2017, 27–28 March 2017 London, United Kingdom. Orphan Drugs Summit, 21-22 September,2017 Netherlands.7th International Meeting on Pulmonary Rare Disease and Orphan Drugs, 24-25 February, Milan, Italy.
Related Societies:
Track 10: Living with a Rare Disease
Uncommon maladies are a different heterogeneous gathering of sicknesses with a little in like way beside of their abnormality influencing with affecting the people. 80% of uncommon illnesses have recognized genetic origins and others may have some biological factors, there are wide and a few which are yet to be analysed. Some uncommon illnesses are gained while some impacted people go about as Diseases inspected. Notwithstanding anomaly, they speak to a critical medicinal and medical issue due to their event. For different uncommon maladies have no treatment, however in the event that it exists and if began on time as accessibly accessible to patients, there is a decent guess for them to be capable for typical life. The issues of patients reflected and affected by uncommon maladies are related to the nonappearance of conclusion and convenient experiencing and their treatment or balancing activity.
Related Conferences:
Drug Discovery 2017, 27–28 March 2017 London, United Kingdom. Orphan Drugs Summit, 21-22 September, 2017 Netherlands.7th International Meeting on Pulmonary Rare Disease and Orphan Drugs, 24-25 February, Milan, Italy.11th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, Feb 27- 28, 2017 Amsterdam, Netherlands.10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK.5th International Conference and Exhibition on Pharmacology and Ethnopharmacology, March 23-25, 2017 Orland, USA.6th Global Experts Meeting on Cardiovascular Pharmacology and Cardiac Medications, April 13-14, 2017 Dubai, UAE.
Related Societies:
Track 11: Ethical Issues on Rare Diseases
A universal research moral predominantly focusses on shielding singular members from potential mischief amid the exploration methods. Moral issues of need setting for research subsidizing are for the most part not been an issue of dialog in the bioethics wrangle about. The clashing good commitments of advantage and distributive equity clearly request altogether different levels of subsidizing for rare medication inquire about. Both sorts of vagrant infections—uncommon ailments and tropical illnesses—posture diverse moral difficulties to inquiries of assigning exploration reserves. For both of them the contention between standards of distributive equity in view of utilitarian or lawful rights and standards of usefulness in view of social or good commitments is at the front line.
Related Conferences:
7th International Meeting on Pulmonary Rare Disease and Orphan Drugs, 24-25 February, Milan, Italy. 11th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, Feb 27- 28, 2017 Amsterdam, Netherlands.10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK. Orphan Drugs and Rare Disease Europe, 15 -16 May, 2017 Berlin, Germany. Drug Discovery 2017, 27–28 March 2017 London, United Kingdom. Orphan Drugs Summit, 21-22 September, 2017 Netherlands.
Related Societies:
Track 12: Drug Approvals for Rare Diseases
Orphan drugs are not withstanding for lesser level of risk for wellbeing, adequacy and strength, FDA is energetically tolerating to be adaptable for their innovative work approach not being stringent as in different cases. Since the patient ailment qualities are truly exceptionally one of a kind the stringency is discarded to profit the patient results. The administrative deterrents are regularly handled with key making arrangements for patient enlistments and through the distinguishing proof of result measures that reflect effective varieties in ailment appearances. Vagrant Drugs more often than not keep to an indistinguishable formative ways from other pharmaceutical items where centre lies around testing PK/PD variables, dosing, steadiness, security, adequacy and power. In any case, certain relaxations are given as far as factual documentations like clinical trial of medication hopeful on 1000 subjects amid stage III clinical trials. Relating to market scope restrictions the administration mediations are an additional energy towards creating vagrant medications. Rare medication directions appreciate benefits as assessment motivators, patent securities, showcase selectiveness, and clinical research sponsorships.
Related Conferences:
5th International Conference and Exhibition on Pharmacology and Ethnopharmacology, March 23-25, 2017 Orland, USA. 6th Global Experts Meeting on Cardiovascular Pharmacology and Cardiac Medications, April 13-14, 2017 Dubai, UAE. 2nd World Congress on Rare Diseases and Orphan Drugs, June 29-30, 2017 London, UK. 6th World Pharmacists & Clinical Pharmacy Annual Congress, May 22-23, 2017 | Chicago, USA. International Conference and Exhibition on Nano medicine and Drug Delivery May 29-31, 2017 Osaka, Japan.
Related Societies:
Track 13: Patient Concerns for Orphan Drugs
Orphan drugs are vaccines or antibodies proposed to treat, avoid or analyse an orphan disease. Cases of rare ailments incorporate genetic diseases, uncommon tumours, irresistible tropic ailments and degenerative infections. The meaning of uncommon ailments shifts crosswise over wards yet commonly considers ailment commonness, seriousness and presence of alternative therapeutic options.
Related Conferences:
7th European Bio-similars Congress May 15-17, 2017 Munich, Germany. 6th World Pharmacists & Clinical Pharmacy Annual Congress, May 22-23, 2017 | Chicago, USA. International Conference and Exhibition on Nano medicine and Drug Delivery, May 29-31, 2017 | Osaka, Japan. 2nd International Conference and Exhibition on Marine Drugs & Natural Products, June 15-17, 2017 | London, UK.
Related Societies:
Track 14: Gaming and Abuse of Orphan Drugs
The 1983 Orphan Drug Act was planned to give tranquilize organizations liberal motivators to create items that may not generally appear to be beneficial ventures. Organizations regularly apply for a vagrant medication assignment ahead of schedule in the medication advancement handle. On the off chance that a vagrant medication is at last endorsed for the market, the FDA postpones the US$2.17-million 'client charge' that organizations must pay to the FDA for new medications. The organizations additionally get impose credits for caused clinical-trial costs, alongside seven years of market restrictiveness, amid which time endorsements for comparative medications are blocked.
Related Conferences:
Orphan Drugs and Rare Disease Europe, 15 -16 May, 2017 Berlin, Germany. Drug Discovery 2017, 27–28 March 2017 London, United Kingdom. Orphan Drugs Summit, 21-22 September, 2017 Netherlands. 7th International Meeting on Pulmonary Rare Disease and Orphan Drugs, 24-25 February, Milan, Italy. 11th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, Feb 27- 28, 2017 Amsterdam, Netherlands.10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 13-15, 2017 London, UK.
Related Societies:
Track 15: Entrepreneurs Investment Meet
A stage meant to associate Entrepreneurs, Proposers and the Investors around the world. It's expected to make and encourage the most advanced and reasonable meeting place for drawing in individuals in worldwide business talks, assessment and execution of promising business thoughts. A financial specialist might discover the most noteworthy potential speculation openings universally, which give great degree of profitability. For business people, this would be a perfect place to discover reasonable speculators and accomplices to begin as well as grow their business. In this way it is a flawless place to associate Entrepreneurs, Business Owners, Early Stage Companies and Established Corporates with National or International Investors, Corporate Investors and Potential Business Partners.
Related Conferences:
7th European Bio-similars Congress May 15-17, 2017 Munich, Germany. 6th World Pharmacists & Clinical Pharmacy Annual Congress, May 22-23, 2017 | Chicago, USA. International Conference and Exhibition on Nano medicine and Drug Delivery, May 29-31, 2017 | Osaka, Japan. 2nd International Conference and Exhibition on Marine Drugs & Natural Products, June 15-17, 2017 | London, UK.
Related Societies:

Greetings!
It is my pleasure to welcome you to San Antonio, TX for the "Annual Congress on Orphan Drugs and Rare Diseases". Rare disorders affect over 350 Million people globally – which means if all people affected by one of ~7000 rare disorders are put together in a country, that would be the third most populous country in the world.
In the United States, we have over 30 Million patients suffering from these debilitating rare diseases. About 80% of the rare diseases are estimated to be genetic in origin and a half of those are early onset before 5 years of age. In 1983, US became the first country to adopt an orphan drug act (ODA) that incentivized biopharmaceutical industry to invest in research and development of orphan drugs. Some key incentives include: tax breaks on R&D expenses incurred on orphan drugs/products, seven years of market exclusivity on orphan drugs in USA even without other intellectual property (IP) protection, among other allowances.
We are in an exciting era of Precision Medicine with a growing number of orphan drugs approved and in the pipeline. Of the ~3,800 orphan drug designations issued by FDA OOPD since 1983, about 600 have resulted in marketing approval, the vast majority with orphan exclusivity. EU, Japan and several countries have adopted some form of ODA over the decades yet many countries are lagging behind – including the two most populous countries in the world – China and India.
Adopting a standard and consistent definition of rare disease is a critical pre-requisite milestone for a nation so that everything else can follow – including a national health policy, database of rare diseases, orphan designation, budget decisions, etc. The Organization for Rare Diseases India (ORDIndia) has played a vital catalytic role in accelerating the pace of progress by unifying all disease specific patient advocacy groups together to represent the collective voice of all stakeholders to the Government policy makers. India now has a definition: A disease is rare if it affects less than 1 in 2500 people. With the number of patients with rare diseases living in India estimated at over 70 million, getting them proper diagnosis and connecting them with global clinical studies is a big goal for ORDIndia.
A number of international consortia and organizations have sprung up recently to support the rare disease revolution and for sharing of best practices for national umbrella organizations. These include: the international rare disease research consortium (IRDiRC), Rare Diseases International (RDI), International Conference on Rare Diseases and Orphan Drugs (ICORD), the NGO committee for rare diseases by United Nations. Numerous regional alliances are also being formed particularly in Asia including the Asia Pacific Alliance for Rare Disease Organizations (APARDO) and Rainbow Across Borders. Conferences such as this one and the world orphan drugs congress where global organizations converge enable exchange of ideas, innovations and best practices benefit the cause of patients with rare diseases globally.
Whether you are a patient advocate, scientist, technologist, student, entrepreneur, pharmaceutical executive, biotech investor, or philanthropist, I truly hope you find this conference very informative and help you gain global insights for local action to continue in your own journey in this exciting area of rare diseases and orphan drugs.

Conference Series invites all the participants across the globe to attend “Annual Congress on Emerging Orphan Drugs and Rare Diseases” during October 30-November 01, 2017 San Antonio, USA which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions.
Orphan Drugs 2017 is an International Rare Diseases Conference, encompassing genetically caused rare diseases and fundamental research and development in respective field. It is a Global platform reaching across all the rare diseases around the globe, covers research and developments of new treatments. The conference attains significance when we look at the worldwide deaths due to Rare Diseases, about 30 percent of children with rare diseases will die before reaching their fifth birthday
Why to attend???
Join your peers around the world focused on learning about Orphan Drugs and related advances, which is your single best opportunity to reach the largest assemblage of participants from the Orphan Drugs community, conduct demonstrations, distribute information, meet with current and potential professionals, make a splash with a new research works, and receive name recognition at this 2-day event. World-renowned speakers, the most recent research, advances, and the newest updates in Orphan Drugs are hallmarks of this conference.
Target Audience:
-
Students
-
Professors & Faculty Members
-
Comapany CEOs & Founders
-
Research Groups
-
Start- Ups
-
Regulatory Authority Representative
-
Patent Attorneys
-
BD Managers
-
Directors, Presidents, VP
-
Industry Research Scientists & CSO
Orphan drugs, also called niche busters, are intended to treat patients suffering from very serious diseases for which no treatment or at least a satisfactory one has so far been available. These diseases, often referred to as orphan diseases or rare diseases, affect only a small portion of the population .The number of diseases for which no treatment is currently available is estimated to be between 4,000 to 5,000, worldwide. Orphan drugs are medicinal products which are used for the treatment of diseases or conditions which affect a very small portion of the population which are known to be rare diseases Like Infectious diseases, Genetic Diseases and Etc.
The Global Orphan Drug Market was around US$97 Billion in 2014, and is anticipated to grow to around US$181.4 Billion by 2020.It is believed that, the market will continue the trend of its incessant growth owing to factors, such as inclining healthcare expenditure, increasing prevalence of chronic diseases, short timeline required for orphan drug development. Moreover, the Market Analysis report includes the revenue of top 10 orphan drugs in 2014. Worldwide, the orphan drug market reached $84.9 billion in 2009. The market is expected to grow at a compound annual growth rate (CAGR) of nearly 6% to reach $112.1 billion by 2014. The U.S. accounted for 51% of the market in 2009 and is expected to grow at a CAGR of 8.9% to reach $65.9 billion by 2014. Orphan drugs for the cancer sector generated the largest amount of revenues, $30.6 billion in 2009, and accounting for 36% of the market. Revenues for cancer-related orphan drugs are expected to grow at a CAGR of 10% to reach $49.7 billion in 2014..According to the following market analysis report, Global Orphan Drugs Market Outlook 2020, in the wake of the above developments the Global Orphan Drug Industry is expected to increase at a CAGR of around 11% from 2014-2020.
Various drivers and challenges have been listed down that affect the Global Orphan Drug Industry. We have also deduced a list of top five companies which are ruling this industry. These companies include the likes of Novartis, Roche, Celgene, Pfizer and Sanofi.
For more details please visit http://market-analysis.conferenceseries.com/orphandrugs-market-reports
Market Penetration
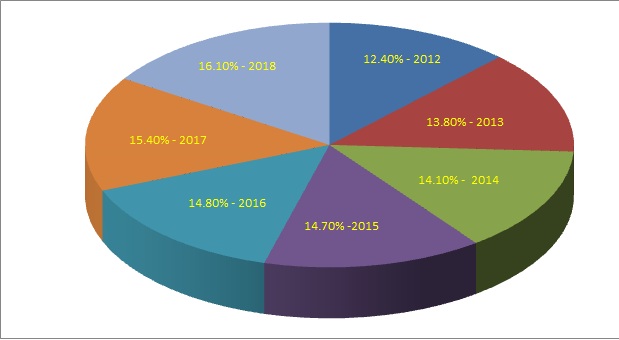
PERCENTAGE SHARE OF ORPHAN DRUGS SALES IN THE PRESCRIPITION DRUGS MARKET 2012 - 2018
Importance & Scope:
There are more than 7,000 classified rare diseases and 70% of them have no type of treatment, there are extensive neglected needs around there. Administrative advantages, for example, longer market selectiveness, leap forward assignments, diminished expenses and assessment motivators are all reassuring speculation. In any case, the promoting procedure and lifecycle for an uncommon infection medication are altogether different to a mass market item and require specific abilities and information.
Orphan Drugs 2017 will be the best stage for every one of the experts and super pros, eminent Scientists, inquire about researchers, understudies who are working in this field over the globe under a solitary rooftop to trade their insight identified with Rare Diseases and Orphan Drugs
Why San Francisco:
Orphan Drugs are a complex group of heterogeneous maladies and are known for their irregularity. Just around 25% of uncommon diseases identified have their molecular basis characterized. There a numerous difficulties extending from illness acknowledgment to conclusion and advancement of new medication.
San Francisco (SF) officially the City and County of San Francisco, is the cultural, commercial, and financial centre of Northern California and the only consolidated city-county in California. San Francisco is about 47.9 square miles (124 km2) in area. It is located on the north end of the San Francisco Peninsula. It is the smallest county in the state. It has a density of about 18,451 people per square mile (7,124 people per km2), making it the most densely settled large city (population greater than 200,000) in the state of California and the second-most densely populated major city in the United States after New York City. San Francisco is the fourth-most populous city in California, after Los Angeles, San Diego, and San Jose, and the 13th-most populous city in the United States. The city and its surrounding areas are known as the San Francisco Bay Area, and are a part of the larger OMB-designated San Jose-San Francisco-Oakland combined statistical area, the fifth most populous in the nation with an estimated population of 8.7 million.
San Francisco State University is part of the California State University system and is located near Lake Merced. The school has approximately 30,000 students and awards undergraduate, masters and doctoral degrees in more than 100 disciplines. The City College of San Francisco, with its main facility in the Ingleside district, is one of the largest two-year community colleges in the country. It has an enrolment of about 100,000 students and offers an extensive continuing education program.
Universities associated with rare diseases research:
North-western University
University of California
University of Pennsylvania
University College London
St George’s University of London
Cambridge University
Feinberg School of Medicine
Oxford University
Imperial College London
King’s College London
Queen Mary University London
Conference Highlights:
|
Overview on Rare Diseases |
|
Types of Rare Diseases |
|
Genetic Rare Diseases |
|
Challenges in Rare Diseases |
|
Orphan Products |
|
Orphan drug: Development trends and strategies |
|
Living with a Rare Disease |
|
Clinical Research: Rare Diseases |
|
Ethical Issues: Rare Diseases |
|
Drug Approvals for Rare Diseases |
|
Orphan Drugs Pricing and Reimbursement |
|
Market Forecasts for Orphan Drugs |
|
Patient Concerns for Orphan Drugs |
|
Gaming and Abuse of Orphan Drugs Market Analysis for Orphan Drugs |
|
Entrepreneurs Investment Meet |
Target Audience:
Directors, CEO’s of Organizations
Business Development Managers
Chief Scientific Officers
PhD Scholars
Patent Attorneys
Intellectual Property Attorneys
Investment Analysts
Association, Association presidents and professionals
Noble laureates in Health Care and Medicine
Research Institutes and members
Supply Chain companies
Manufacturing Medical Devices Companies
Faculty of Pharmaceutical Universities
Students
Medical Colleges
Why to attend?
In today's economic atmosphere your business choices are as crucial as ever. "Global Summit on Emerging Orphan Drugs and Drug Abuse” is permit you to amplify your time and advertising dollars while getting quick criticism on your new items and administrations. Global Summit on Emerging Orphan Drugs and Drug Abuse is sorting out an extraordinary Scientific Exhibition/Program and expects the world's driving authorities included Rare Diseases and Orphan Drugs. Your association will profit with great presentation to the pioneers in Rare Diseases and Orphan Drugs. Orphan Drugs-2017 is an energizing chance to showcase the new innovation, the new results of your organization, as well as the administration your industry may offer to a wide universal group of audience.
Research Associations related to rare diseases:
Organisation for Rare Diseases India
The Genetic and Rare Disorders Organization
Canadian Organisation for rare diseases
The Greek Alliance for Rare Diseases
The Every Life Foundation for Rare Diseases
Global Genes Project
National Organisation for rare diseases
The Asia-Pacific Alliance of Rare Disease Organisations
EURORIDS rare diseases Europe
Rare Diseases Translational Research Collaboration
Market Analysis Report:
Orphan Drugs are kind of pharmaceutical drugs that are utilized as a part of the treatment of rare genetic diseases or uncommon therapeutic conditions. The worldwide pharmaceutical medications market was esteemed at around $962.1 billion in 2012, which is the parent advertise for orphan drugs. FDA and European Commission are included in giving unique kind of convention to organizations that are encountering business hindrances, for example, item rivalry and inventory network barriers. The aggregate number of infections arranged as orphan stands at around 7,000 at present, with almost 250 new maladies being annexed to this rundown every year
In 2016, orphan drug sales expanded by 8.5% to $85 billion from the earlier year. That contrasts and a 2.1 % decrease in general physician endorsed medicate deals (barring generics), which tumbled to $645 billion. Kyprolis, a medication from Onyx Pharmaceuticals for numerous myeloma, was the most encouraging new vagrant medication in 2012, with U.S. deals anticipated that would reach $897 million in 2017. With the overall orphan drug market set to reach $127 billion by 2018, representing about 16% of aggregate physician recommended sedate deals, it's turning into the following field for some of pharma's greatest leaps forward and fiercest patent wars. FDA market research analyst has estimated the global orphan drugs market to exhibit a moderate growth rate during the forecast period and is envisaged to surpass USD 156 billion by 2019.
The Orphan Products Clinical Trials Grants Program has been accustomed to convey more than 55 items to advertising endorsement. Awards guarantee that item advancement happens in an opportune way with an extremely unobtrusive speculation. As a rule, OOPD allow subsidizing goes on for three-four years. At any one time, there are regularly 60 to 85 on-going stipend supported activities. A noteworthy part of the appropriated stores (ordinarily roughly $15 million) for a given monetary year go towards kept financing of earlier endorsed gifts. The fast increment in the cost of clinical trials as of late has blocked an expansion in the quantity of new OOPD awards. OOPD normally subsidizes 12-18 new concedes per financial year.
Top 20 orphan drugs by 2018
- Rituxan
- Revlimid
- Soliris
- Afinitor
- Tasigna
- Velcade
- Avonex
- Alimta
- Yervoy
- Sprycel
- Rebif
- Kalydeco
- Jakavi
- Sutent
- Kyprolis
- Kogenate
- NovoSeven
- Nexavar
- Copaxone
- Ibrutinib
Conference Highlights
- Market Analysis for Orphan Drugs
- Orphan Drugs Pricing and Reimbursement
- Challenges in Rare Disease
- Overview on Rare Diseases
- Living with a Rare Disease
- Drug Approvals for Rare Diseases
- Entrepreneurs Investment Meet
- Clinical Research On Rare Diseases
- Ethical Issue On Rare Diseases
- Patient Concerns for Orphan Drugs
- Development Trends and Strategies On Orphan Drugs
- Genetic Rare Diseases
- Types of Rare Diseases
- Orphan Products
- Gaming and Abuse of Orphan Drugs
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | October 30-31, 2017 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Developing Drugs
- International Journal of Drug Development and Research
- Journal of Clinical Trials
Abstracts will be provided with Digital Object Identifier by


